Water in its liquid form has an unusually high boiling point temperature, a value close to 100°C. A considerable amount of heat energy is required to accomplish this change in water. As liquid water heats up, hydrogen bonding makes it difficult to separate the water molecules from each other, which is required for it to enter its gaseous phase . The formation of hydrogen bonds is an important quality of liquid water that is crucial to life as we know it. In liquid water, hydrogen bonds are constantly formed and broken as the water molecules slide past each other. The breaking of these bonds is caused by the motion of the water molecules due to the heat contained in the system.
On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding . This makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids. Conversely, as molecular motion decreases and temperatures drop, less energy is present to break the hydrogen bonds between water molecules.
These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) (Figure 2.8 a). This means that ice floats on the surface of a body of water (Figure 2.8 b). In lakes, ponds, and oceans, ice will form on the surface of the water, creating an insulating barrier to protect the animal and plant life beneath from freezing in the water. If this did not happen, plants and animals living in water would freeze in a block of ice and could not move freely, making life in cold temperatures difficult or impossible. While groundwater usually has low DO levels, groundwater-fed streams can hold more oxygen due to the influx of colder water and the mixing it causes ¹⁵.
Does Air And Water Signs Mix While water equilibrates toward 100% air saturation, dissolved oxygen levels will also fluctuate with temperature, salinity and pressure changes ³. As such, dissolved oxygen levels can range from less than 1 mg/L to more than 20 mg/L depending on how all of these factors interact. In freshwater systems such as lakes, rivers and streams, dissolved oxygen concentrations will vary by season, location and water depth. Historically the Bay's oyster population was in the tens of billions, and they circulated the entire Bay volume in a matter of days. Due to pollution, disease and over-harvesting their population are a fraction of their historic levels. Water that was once clear for meters is now so turbid and sediment ridden that a wader may lose sight of their feet before their knees are wet.
Oxygen is normally supplied by "Submerged Aquatic Vegetation" via photosynthesis but pollution and sediments have reduced the plant population as well. Resulting in a reduction of dissolved oxygen levels rendering areas of the bay unsuitable for aquatic life. Researchers have proposed that oxygenation through artificial means as a solution to help improve water quality. Aeration of hypoxic water bodies seems an appealing solution and it has been tried successfully many times on freshwater ponds and small lakes. However no one has undertaken an aeration project as large as an estuary. The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances.
As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules.
Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates temperature changes within organisms and in their environments. As energy input continues, the balance between hydrogen-bond formation and destruction swings toward the destruction side.
This process results in the release of individual water molecules at the surface of the liquid in a process called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body. During a long hot summer, you may notice fish gulping air at the surface of a pond.
Why do you think the fish come to the surface like this, instead of breathing dissolved oxygen in the water the way they normally do? Like carbon dioxide, the concentration of dissolved oxygen is also affected by temperature. Cold water can hold more dissolved oxygen than warm water.
In winter and early spring, when the water temperature is low, the dissolved oxygen concentration is high. In summer and early fall, when the water temperature is high, the dissolved-oxygen concentration is lower.Coal-burning power plants heat water to turn turbines to make electricity. After using the water, it is cooled and then returned to the river or lake it came from.
Why is it important to cool the water before returning it to the river? Dissolved gases, like oxygen for fish and carbon dioxide for aquatic plants, would escape if the returned water were hot. The equation shows that water will remain at 100% air saturation at equilibrium. However, there are several factors that can affect this. Aquatic respiration and decomposition lower DO concentrations, while rapid aeration and photosynthesis can contribute to supersaturation. During the process of photosynthesis, oxygen is produced as a waste product.
This adds to the dissolved oxygen concentration in the water, potentially bringing it above 100% saturation ¹⁴. In addition, the equalization of water is a slow process . This means that dissolved oxygen levels can easily be more than 100% air saturation during the day in photosynthetically active bodies of water ¹⁴. The capability for a molecule to absorb heat energy is called heat capacity, which can be calculated by the equation shown in the figure.
Water's high heat capacity is a property caused by hydrogen bonding among water molecules. When heat is absorbed, hydrogen bonds are broken and water molecules can move freely. When the temperature of water decreases, the hydrogen bonds are formed and release a considerable amount of energy. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. As a result, it takes water a long time to heat and a long time to cool.
In fact, the specific heat capacity of water is about five times more than that of sand. Many tropical saltwater fish, including clown fish, angel fish and groupers require higher levels of DO, such as those surrounding coral reefs. Coral reefs are found in the euphotic zone (where light penetrates the water – usually not deeper than 70 m). Higher dissolved oxygen concentrations are generally found around coral reefs due to photosynthesis and aeration from eddies and breaking waves ³⁷. These DO levels can fluctuate from 4-15 mg/L, though they usually remain around 5-8 mg/L, cycling between day photosynthesis production and night plant respiration ³⁸. In terms of air saturation, this means that dissolved oxygen near coral reefs can easily range from % ³⁹.
Not all water depths reach 100% air saturationIn a stable body of water with no stratification, dissolved oxygen will remain at 100% air saturation. 100% air saturation means that the water is holding as many dissolved gas molecules as it can in equilibrium. At equilibrium, the percentage of each gas in the water would be equivalent to the percentage of that gas in the atmosphere – i.e. its partial pressure ¹³.
The water will slowly absorb oxygen and other gasses from the atmosphere until it reaches equilibrium at complete saturation 10. This process is sped up by wind-driven waves and other sources of aeration ³. At the macroscopic level, heat is the transfer of energy from the high temperature object to the low temperature object. At the particle level, heat flow can be explained in terms of the net effect of the collisions of a whole bunch of little bangers. Warming and cooling is the macroscopic result of this particle-level phenomenon. Now let's apply this particle view to the scenario of the metal can with the hot water positioned inside of a Styrofoam cup containing cold water.
On average, the particles with the greatest kinetic energy are the particles of the hot water. Being a fluid, those particles move about with translational kinetic energy and bang upon the particles of the metal can. As the hot water particles bang upon the particles of the metal can, they transfer energy to the metal can. Most metals are good thermal conductors so they warm up quite quickly throughout the bulk of the can. The can assumes nearly the same temperature as the hot water.
Being a solid, the metal can consists of little wigglers. The wigglers at the outer perimeter of the metal can bang upon particles in the cold water. The collisions between the particles of the metal can and the particles of the cold water result in the transfer of energy to the cold water.
The interaction between the particles of the hot water, the metal can and the cold water results in a transfer of energy outward from the hot water to the cold water. At the macroscopic level, one would observe a decrease in temperature of the hot water and an increase in temperature of the cold water. If you have been following along since the beginning of this lesson, then you have been developing a progressively sophisticated understanding of temperature and heat.
You should be developing a model of matter as consisting of particles which vibrate , translate and even rotate . Temperature is a measure of the average amount of kinetic energy possessed by the particles in a sample of matter. The more the particles vibrate, translate and rotate, the greater the temperature of the object. You have hopefully adopted an understanding of heat as a flow of energy from a higher temperature object to a lower temperature object. It is the temperature difference between the two neighboring objects that causes this heat transfer. The heat transfer continues until the two objects have reached thermal equilibrium and are at the same temperature.
The discussion of heat transfer has been structured around some everyday examples such as the cooling of a hot mug of coffee and the warming of a cold can of pop. Finally, we have explored a thought experiment in which a metal can containing hot water is placed within a Styrofoam cup containing cold water. Heat is transferred from the hot water to the cold water until both samples have the same temperature. Sockeye salmon with gas bubble diseaseJust as low dissolved oxygen can cause problems, so too can high concentrations. Supersaturated water can cause gas bubble disease in fish and invertebrates ¹². Significant death rates occur when dissolved oxygen remains above 115%-120% air saturation for a period of time.
Total mortality occurs in young salmon and trout in under three days at 120% dissolved oxygen saturation ¹². Invertebrates, while also affected by gas bubble disease, can usually tolerate higher levels of supersaturation than fish ¹². Warm, shallow saltwater reaches 100% air saturation at a lower concentration, but can often achieve levels over 100% due to photosynthesis and aeration.
Shallow waters also remain closer to 100% saturation due to atmospheric contact and constant diffusion ¹⁰. When you looked closely, you might have observed that on the graphite electrode connected to the negative pole of the battery more gas was formed than on the other side. Collecting the two gases with the jumbo straws probably demonstrated this even better. The difference is due to the fact that one water molecule has two hydrogen atoms to one oxygen atom, as explained above. This means it takes two water molecules to make one oxygen molecule . At the same time, however, two molecules of water can make two molecules of hydrogen .
Whereas hydrogen and oxygen are formed at the electrodes, the leftover reaction products from water are protons (H+ on the side of oxygen) and hydroxyl ions (OH- on the side of hydrogen). You can visualize this by putting a pH strip into the solutions in the jumbo straws above each electrode. The solution in the straw that was put on top of the negative battery pole electrode should show a basic pH whereas the other one should be acidic . Water has many properties that are critical to maintaining life.
It is polar, allowing for the formation of hydrogen bonds, which allow ions and other polar molecules to dissolve in water. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. Water's cohesive forces allow for the property of surface tension. All of these unique properties of water are important in the chemistry of living organisms. If the hypolimnion is deep enough to never mix with the upper layers, it is known as the monimolimnion.
The hypolimnion is separated from the upper layers by the chemocline or halocline. These clines mark the boundary between oxic and anoxic water and salinity gradients, respectively. While lab conditions would conclude that at colder temperatures and higher pressures water can hold more dissolved oxygen, this is not always the result. In the hypolimnion, bacteria and fungi use dissolved oxygen to decompose organic material ⁶. This organic material comes from dead algae and other organisms that sink to the bottom.
The dissolved oxygen used in decomposition is not replaced – there is no atmospheric contact, aeration or photosynthesis to restore DO levels in the hypolimnion ¹¹. Thus the process of decomposition "uses up" all of the oxygen within this layer. Extended periods of supersaturation can occur in highly aerated waters, often near hydropower dams and waterfalls, or due to excessive photosynthetic activity. Algae blooms can cause air saturations of over 100% due to large amounts of oxygen as a photosynthetic byproduct. This is often coupled with higher water temperatures, which also affects saturation.
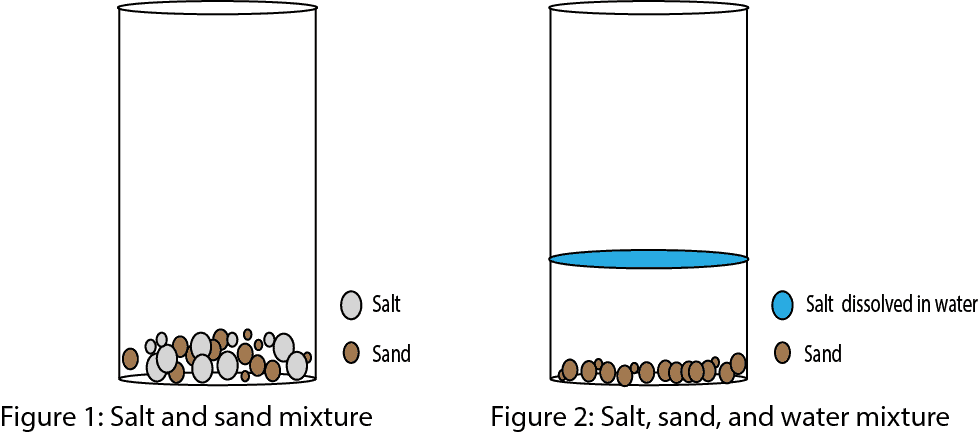
¹² At higher temperatures, water becomes 100% saturated at lower concentrations, so higher dissolved oxygen concentrations mean even higher air saturation levels. In general, dissolved oxygen levels are about 20% less in seawater than in freshwater ³. Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent—a substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water.
In the case of table salt mixed in water, the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems. To calculate dissolved oxygen concentrations from air saturation, it is necessary to know the temperature and salinity of the sample.
Barometric pressure has already been accounted for as the partial pressure of oxygen contributes to the percent air saturation 7. Salinity and temperature can then be used in Henry's Law to calculate what the DO concentration would be at 100% air saturation 10. However, it is easier to use an oxygen solubility chart. These charts show the dissolved oxygen concentration at 100% air saturation at varying temperatures, and salinities.